Our Technology
Sierra CRO
We understand the power of technology, the importance of intuitive reporting and the ability to have real time data at your fingertips. Our team of experts leverage powerful tools, that provide operational efficiency, data-driven decision making and accelerated achievement of study goals. At Sierra, our Data Managers are certified and trained, allowing for the ability to create customized reports and dashboards.


EDC System
Sierra CRO
 We believe in leveraging easy to access EDC software that is intuitive, interactive, efficient and compliant. At Sierra, we have conducted over 50 studies using Merative Zelta™ for electronic data capture. The tools we utilize in our EDC system expedite the data cleaning and database lock processes. This system is a favorite among study sites and sponsors; and is both 21 CFR Part 11 and HIPAA compliant.
We believe in leveraging easy to access EDC software that is intuitive, interactive, efficient and compliant. At Sierra, we have conducted over 50 studies using Merative Zelta™ for electronic data capture. The tools we utilize in our EDC system expedite the data cleaning and database lock processes. This system is a favorite among study sites and sponsors; and is both 21 CFR Part 11 and HIPAA compliant.
Medical Coding Module
Sierra CRO
For a clinical trial to succeed, subject data entered in EDC as verbatim text must be coded. The Zelta EDC system has a powerful Medical Coding module that Sierra leverages to streamline the coding process with accuracy and consistency. This tool offers many efficiencies and includes a coding approval process. MedDRA, WHODrug, and custom (preferred term) dictionaries can be utilized within the module.

Advanced Reporting
Sierra CRO
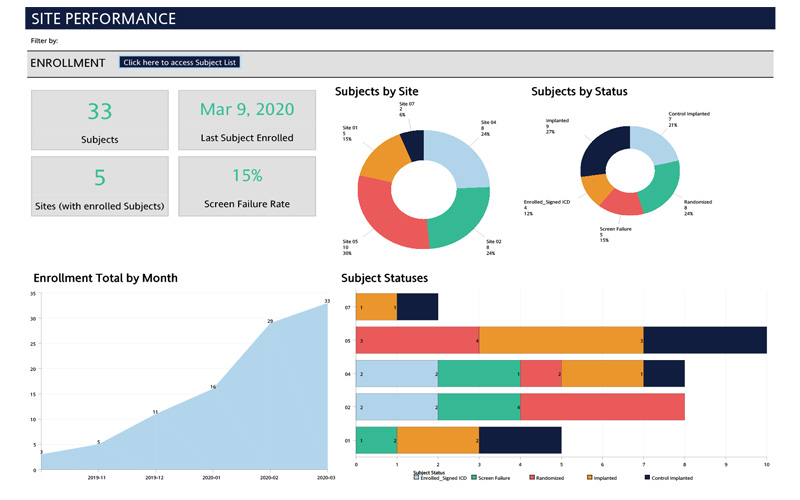
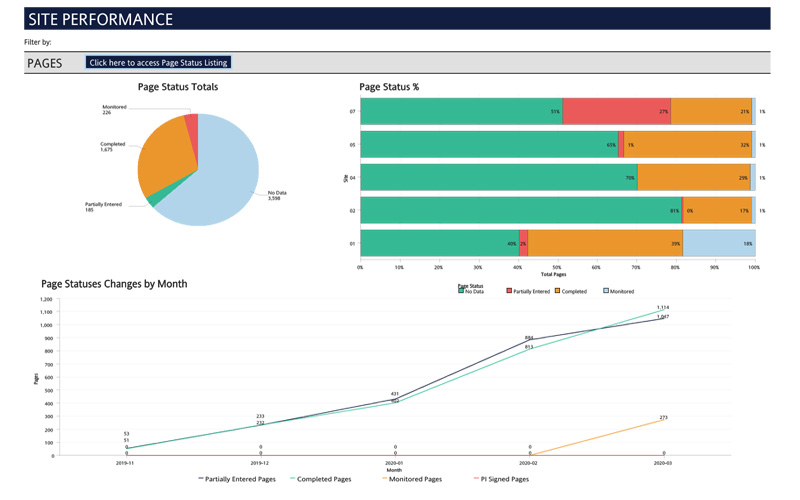
With the Cognos Smart Reports module, Sierra's DM experts have the ability to generate expansive, customized and filtered reports and metrics that can be delivered to designated study team members via email on a routine schedule. Additionally, our experts can create interactive and highly dynamic dashboards that provide extreme efficiency in trial management. Dashboards available include study management, site performance, monitoring/SDV status, safety & compliance management. They provide a bird's-eye view for study oversight with the ability to quickly click on sections to filter the dashboard or drill down to the details.
The Merative Zelta™ EDC system includes a well-rounded set of standard reporting options including visit schedule reports to quickly identify missed and out of window visits, data trackers to help identity outliers, and listings for more detailed data review. Reports and dashboards are designed and customized per project based on request from the sponsor.
Trial Interactive eTMF System by TransPerfect
Sierra CRO
 We streamline TMF compliance with an eTMF that empowers every stakeholder including Sponsors, Project Managers, CTAs, CRAs and DM teams to be more compliant and more efficient in day-to-day operations. We ensure your eTMF is high-quality, complete, and audit-ready at all times with the Trial Interactive system. This validated system is 21 CFR Part 11 compliant.
We streamline TMF compliance with an eTMF that empowers every stakeholder including Sponsors, Project Managers, CTAs, CRAs and DM teams to be more compliant and more efficient in day-to-day operations. We ensure your eTMF is high-quality, complete, and audit-ready at all times with the Trial Interactive system. This validated system is 21 CFR Part 11 compliant.